Turning Protocols Into Action
We don’t just optimize study designs on paper — we ensure sites are prepared to deliver. Our team regularly presents at sponsor investigator meetings, where we walk principal investigators and clinical research coordinators through the recruitment protocol step by step.
At these meetings, we also present results from Patient Advisory Boards and Panels, where patients review the protocol in advance and provide candid feedback on visit schedules, burdens, and potential barriers to enrollment. Sharing these findings directly with investigators ensures sites understand the patient perspective, adopt patient-informed best practices, and are better equipped to engage and retain participants.
Using detailed slide decks and real-world data examples, we:
-
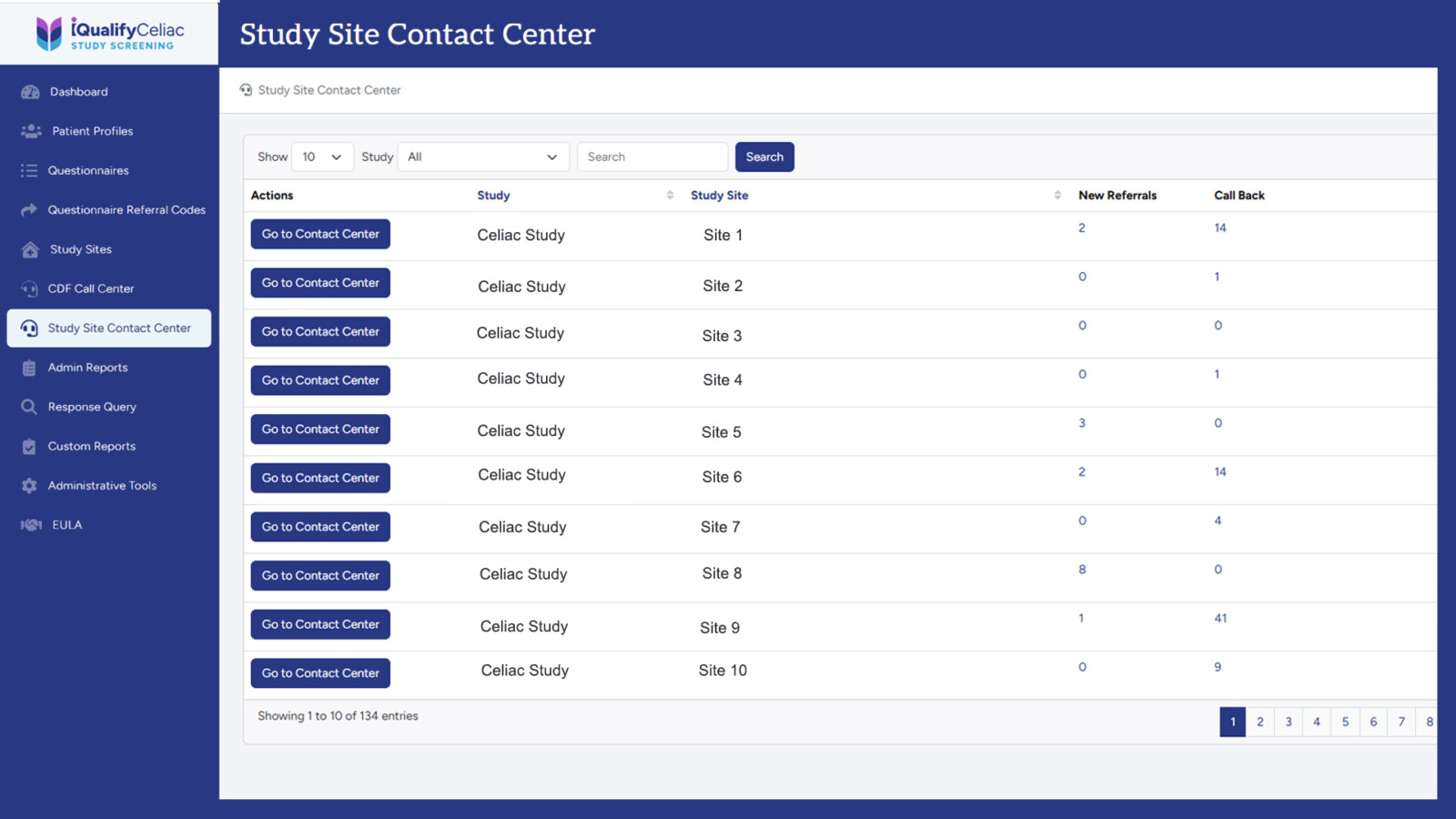
Demonstrate how patient referrals flow from iQualifyCeliac into site workflows.
-
Share coordinator-facing tools for tracking outreach, screen fails, and enrollment outcomes.
-
Highlight best practices for engaging patients and reducing referral drop-off.
-
Provide site-specific performance benchmarks to set clear expectations from the outset.
By combining protocol optimization with proactive training and patient-informed insights at investigator meetings, sponsors gain confidence that sites are aligned, equipped, and motivated to achieve enrollment goals from day one.