iCureCeliac® Patient Registry

iCureCeliac® Global Patient Registry
Verified Patients. Real-World Data. Faster Trials.
The iCureCeliac® Patient Registry is the largest global database of individuals living with celiac disease, with more than 17,000 participants across 90+ countries. Integrated with the Celiac Disease Foundation’s international network, iCureCeliac® provides unparalleled access to both qualified patients for recruitment and comprehensive real-world evidence, giving sponsors and CROs a single source for accelerating treatment development.
Registry-Driven Recruitment
Recruit directly from a verified patient registry rather than relying on broad advertising. iCureCeliac® connects sponsors and CROs with patients who match protocol requirements — significantly reducing screen fails, accelerating enrollment, and easing site burden.
Capabilities include:
- Precision targeting – Match participants using registry demographics, biomarker data, and patient-reported outcomes
- Rapid engagement – Integrated with iQualifyCeliac® for real-time referrals and site-matched workflows
- Global reach – Registry participants across 90+ countries, engaged through trusted advocacy partnerships
- Retention insights – Longitudinal data informs retention strategies and sustained participation
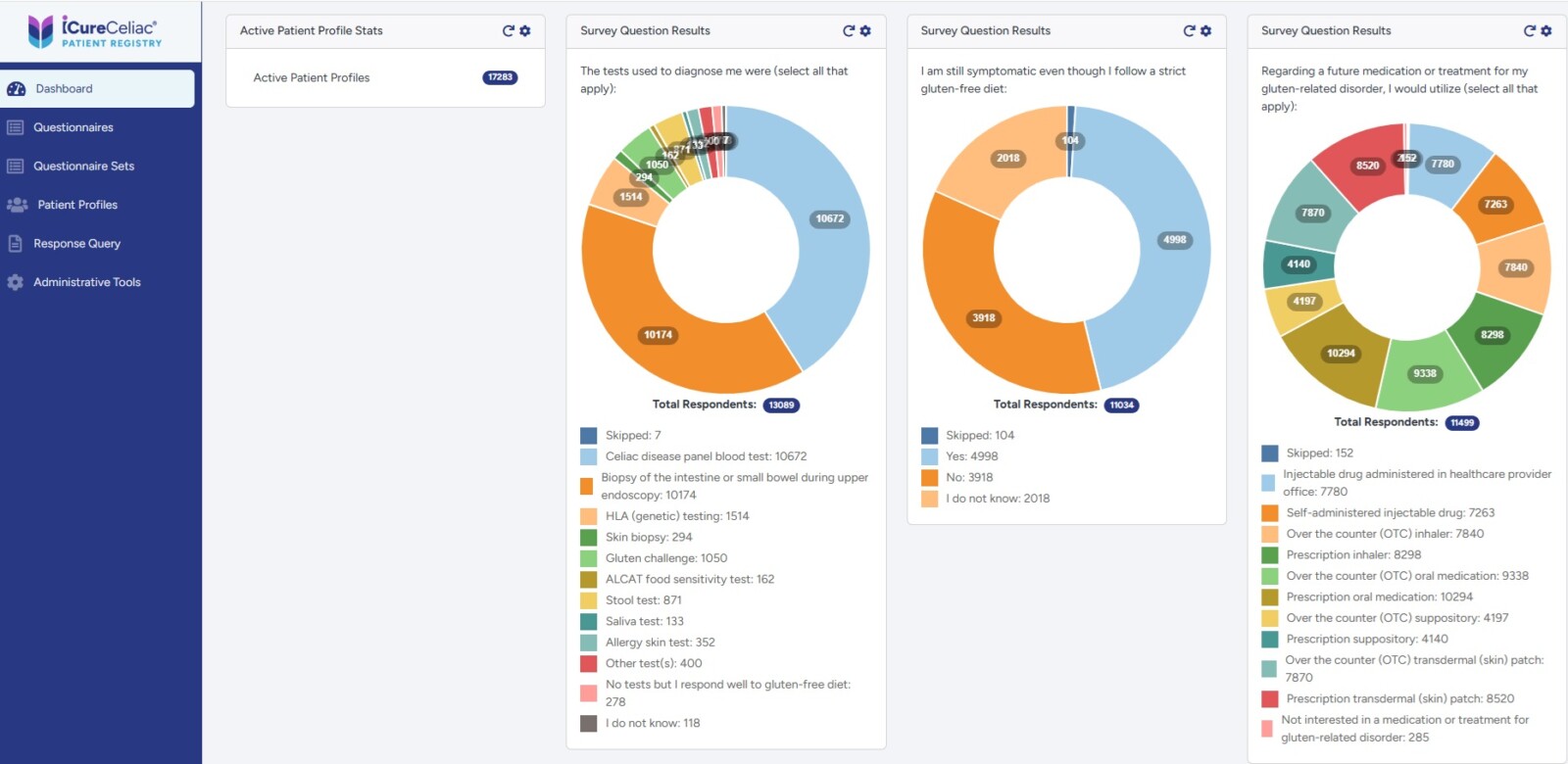
Data Licensing: Real-World Evidence at Scale
iCureCeliac® provides access to comprehensive, longitudinal patient-reported data that strengthens trial design, supports regulatory submissions, and powers high-impact publications.
Key features:
- Comprehensive data sets – demographics, biomarkers, treatment history, comorbidities, healthcare utilization
- Validated instruments – CSI, CDAT, CD-QOL, SF-36, PROMIS for robust, publication-ready outcomes
- Longitudinal insights – tracking disease burden, adherence, and quality of life over time
- Global diversity – patient-reported outcomes from six continents

iCureCeliac®: High-Impact Research
The registry captures the full patient journey — from diagnosis through treatment and disease management. Participants contribute data on the issues that matter most to outcomes, ensuring research reflects authentic experiences and diversity.
Research powered by iCureCeliac® and authored by the Foundation has informed trial design, supported regulatory submissions, and helped secure U.S. federal funding for celiac disease research. Highlights include:
- Economic and societal burden – Analyses of missed work and school days, demonstrating the productivity and quality-of-life impact of celiac disease.
- Quality of life outcomes – Validation of patient-reported outcome tools and studies linking gluten-free diet adherence with mental health and well-being.
- Healthcare disparities – International physician surveys highlighting gaps in diagnosis and patient management.
- Real-world symptom tracking – Virtual symptom studies providing longitudinal data in both adults and adolescents.
- Comorbidity insights – Large-scale analyses of autoimmune conditions and associated health challenges in celiac disease.
Research and Publications
-
Social Adversities Associate with Worse Disease Control in Pediatric Celiac Disease
-
Celiac Disease Symptom Profiles and Their Relationship to Gluten-Free Diet Adherence, Mental Health, and Quality of Life
-
The Virtual Celiac Symptoms Study: Reported Symptoms Over 12 Weeks in Adults
-
The Virtual Celiac Symptoms Study: Reported Symptoms Over 12 Weeks in Adolescents
-
The Virtual Celiac Symptoms Study: Symptom and Gluten-Free Diet Perceptions of Adolescents at Baseline
-
Psychometric Validation of the Celiac Disease-Specific Quality of Life Survey (CD-QOL) in Adults with Celiac Disease in the United States
-
The Virtual Celiac Symptoms Study: Symptom and Gluten-Free Diet Perceptions at Baseline
-
Physician Management of Celiac Disease: A Comparison of Celiac Disease Knowledge, Diagnosis, and Patient Management Between Gastroenterologists and Primary Care Physicians in Germany, Italy, Spain, and the United States – Findings From a Real-World Survey
-
Diagnosing Celiac Disease in the United States of America, Germany, Italy and Spain: Findings From a Real-World Survey
-
Celiac Disease Symptom Flares Are Common During the Postpartum Period
-
Probiotic Use in Celiac Disease: Results from a National Survey
-
Experiences of a Gluten-Free Diet in Patients with Celiac Disease: A Multi-National Survey
-
Disease Burden and Quality of Life Impacts in Patients With Celiac Disease on a Gluten-Free Diet: An Analysis of the iCureCeliac® Registry
-
Prevalence of Dermatitis Herpetiformis in iCureCeliac Patient Registry
-
Patient Burden and Treatment Experience in Celiac Disease
-
Adherence to the Gluten-Free Diet and Celiac Disease Patient Outcomes: Real World Evidences From an International Patient Registry, iCureCeliac®
-
Numbers and Features of Patients With a Diagnosis of Celiac Disease Without Duodenal Biopsy, Based on a National Survey
-
Forgoing the Duodenal Biopsy for Celiac Disease Diagnosis Among Adults in the United States: Results of a National Survey
-
The Effect of Depressive Symptoms on the Association between Gluten-Free Diet Adherence and Symptoms in Celiac Disease: Analysis of a Patient Powered Research Network
-
Autoimmune Diseases and Associated Conditions in Children and Adults with Celiac Disease from the Celiac Disease Foundation’s iCureCeliac® Patient-Powered Research Network
-
Transition From Childhood to Adulthood in Coeliac Disease: The Prague Consensus Report
-
Celiac Disease Clinical Trials: Best Practices White Paper and Toolkit
Unlock the Full Potential of iCureCeliac
Whether your goal is to accelerate enrollment, publish patient-centered research, or strengthen a regulatory submission, iCureCeliac® delivers both the participants and the insights you need.